Brendan
"So it's a hangover cure then?", said Brendan.
Brendan had asked why I'd called my company Catalase , "What did it mean?". I explained that Catalase was an enzyme and that it did a really neat trick - combining dangerous hydrogen peroxide with alcohol, to make water and something useful to the cell.
Brendan looked scandalized, and puzzled, when I talked about it breaking down alcohols.
"Why would anyone want to do that??"
This was an early conversation in one of my first jobs in Ireland. I hadn't yet appreciated the pub culture, the way alcohol is used to break down barriers. You're unfriendly if you don't go to the pub with your colleagues. I'd come from an ultra academic background where the befuddlement that comes from drinking is something to avoid. Fortunately I didn't dig the hole I'd just made for myself any deeper, and it was Brendan who found the common ground, something he could approve of: "So it's a hangover cure then?"
Two Roles
In humans the enzyme most important for dealing with alcohol - and hence hangovers - is Alcohol Dehydrogenase. HP-II (Catalase-peroxidase), which also does break down alcohols, is primarily a bacterial enzyme. My science was a bit muddled. Back when I first learned of Catalase, the textbook I had was not really clear that it was talking about two different kinds.
- HP-I (Catalase) is one of the most efficient enzymes known. According to the textbook, Catalase was this amazingly efficient enzyme, "A reaction with every collision". Catalase is a catalyst that will liberate Oxygen from Hydrogen Peroxide.
- HP-II (Catalase-peroxidase), the bacterial enzyme, is less efficient as a Catalase, but it also converts alcohols. To me the description of its action read as knocking two harmful things together, recombining them, making something harmless, water, and something useful, a precursor to feed into the TCA cycle to generate energy.
It was mind boggling to me that such an efficient enzyme should also have this other role involving alcohols (HP-II role) in addition to its primary role in disproportionating dangerous Hydrogen Peroxide into Oxygen and water (HP-I role).
Amazing Claims
The efficiency and the alcohols role were two different proteins. For me that combination, detoxifying, making useful products, extreme efficiency, which I mistakenly thought was from just one enzyme, was too good a combination to miss. And so I named my company after it.
When I read the textbook I was though very skeptical of the claim 'a reaction with every collision'. A miscalculation surely? If Hydrogen Peroxide approaches Catalase 'from the wrong side', away from the active site, surely it will just bounce off? There are though shapings of enzymes that guide and funnel substrates to the active site, but to do that all the way from the wrong side strained credulity.
I also knew that if you shake a bottle of Hydrogen Peroxide, it will start to fizz without any specific catalyst. The small bubbles that form help to nucleate more release of Oxygen. So it seemed to me it would be very easy to not account for the effect of bubbles 'as a kind of catalyst' and to so think the catalyst you added, Catalase from beef heart, had a far stronger direct effect than it actually did.
Heme
The muddiness of the too-good-to-be-true claims for Catalase don't in the end take away the luster of this molecule, at least not for me. The reason Catalase still shines for me is that it has a truly beautiful molecule at its heart - Heme.
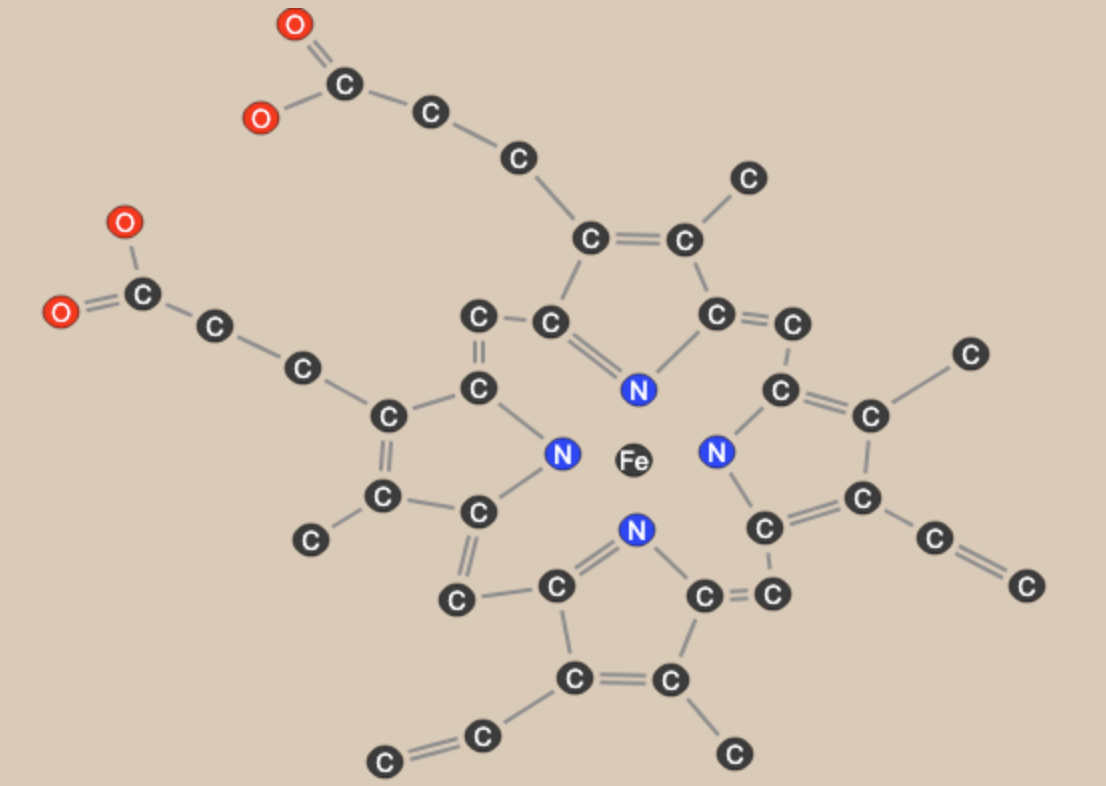
Heme is a prosthetic group to the enzyme. It provides the catalytic site. The protein around the Heme group modulates its activity precisely - steering molecules, holding them in favorable configurations. The same Heme group (or very nearly) shows up in Hemoglobin. There it binds Oxygen to carry it to the organs, and after releasing Oxygen where it is needed, then binds to Carbon Dioxide to carry it away.
Company Name
When I named the company I thought Catalase was one class of protein. Catalase the enzyme is both less extraordinary - the descriptions of Catalase were of two related classes of molecule - and more extraordinary than it seemed then. Today we have far, far more information about proteins' roles than then. The naming of all proteins has perforce become more systematic. The naming nowadays is clearer about what the proteins do. However, I do not feel obliged to rebrand the company as Hydrogen-peroxide Oxidoreductase, or to pivot the company for the opportunities that Brendan foresaw, now as Alcohol Dehydrogenase Limited.